NextUp: The Philly-Area Company Using CBD to Treat Autism
Zynerba Pharmaceuticals is testing a CBD gel for the treatment of behavioral symptoms associated with Fragile X Syndrome, the most common genetic cause of autism.

Armando Anido is the chief executive officer of Zynerba Pharmaceuticals. / Courtesy
“NextUp” is a weekly NextHealth PHL feature that highlights the local leaders, organizations and research shaping the Greater Philadelphia region’s life sciences ecosystem. Email qmuse@phillymag.com with pitches for NextUp.
Who: Zynerba is a clinical-stage pharmaceutical company focused on developing pharmaceutically produced cannabinoid treatments for rare central nervous system disorders. The company has been in existence since 2004, and originally spun out of the University of Kentucky School of Pharmacy under the name AllTranz. In 2007, the company changed its name to Zynerba and established its headquarters in Devon, Pennsylvania.
What: Zynerba has several clinical trials underway to test the efficacy of treatments for several rare and near rare neuropsychiatric disorders, including Fragile X syndrome (FXS), Autism Spectrum Disorder (ASD), 22q (a disorder caused by a small missing piece of the 22nd chromosome), and certain forms of epilepsy, including developmental and epileptic encephalopathies (DEE).
The company recently launched a pivotal clinical trial, CONNECT-FX, for its lead product, Zygel. The trial will evaluate the efficacy and safety of Zygel in 204 children and adolescents ages three through 17 years with Fragile X Syndrome. The trial is enrolling patients in 20 clinical sites in the United States, Australia and New Zealand.
Zygel is the first and only pharmaceutically manufactured CBD that is formulated as a gel with permeation enhancers that allow the drug to be delivered through the skin and into the circulatory system. Unlike THC, the CBD used in Zygel is non-euphoric, meaning it does not create the “high” feeling often associated with cannabis or marijuana.
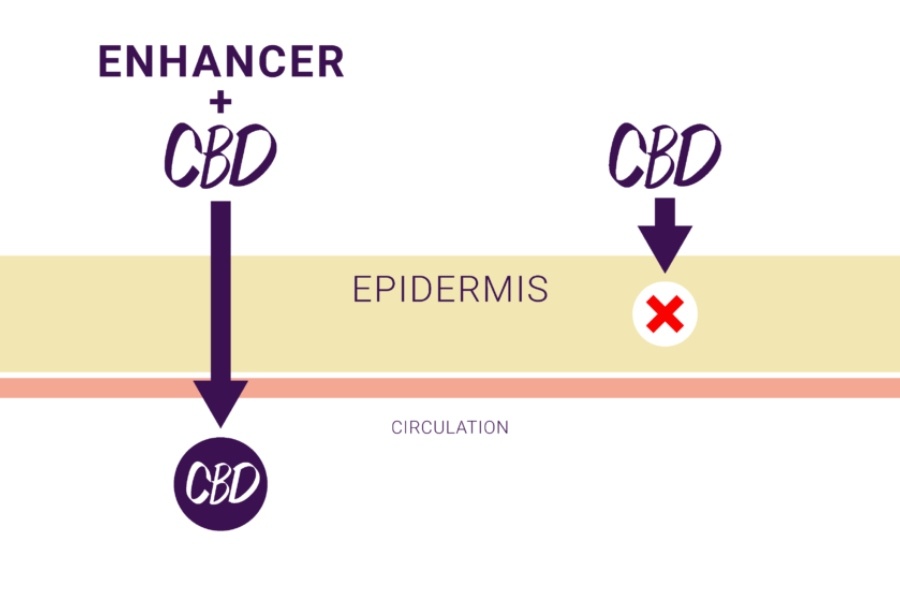
Zynerba’s Zygel features permeation enhancers that enable CBD to be absorbed through the skin and into the bloodstream. / Courtesy
When: In March 2018, the FDA granted orphan drug designation to Zynerba, indicating Zygel as a safe treatment option for patients with FXS. In June 2019, the U.S. Patent and Trademark Office granted Zynerba a second patent for its skin permeating technology. The company hopes to have top-line data from the CONNECT-FX trial in the first half of 2020. According to Zynerba’s chief executive officer Armando Anido, if the trial results are positive, the company hopes to submit a new drug application to the FDA by the end of 2020.
Why: According to estimates, 95 percent of all rare diseases do not have a single FDA approved drug treatment, and FXS is no exception. FXS is a genetic condition that causes a range of developmental problems, including learning disabilities and cognitive impairment. It affects one in every 10,000 youth according to the CDC. There are currently no therapeutic options approved for the treatment of behavioral symptoms associated with FXS.
“When we looked at what’s available and saw that there was nothing available for Fragile X Syndrome, we knew this was a high unmet need,” Anido said. “That’s why we went into these areas. It gives us a nice opportunity to make a huge difference for these patients who have no other treatment options.”
What It Means: Zynerba’s Zygel addresses several key challenges to using cannabinoids to treat CNS disorders in children. It is often difficult to get children to take oral medications. In addition, oral administration of CBD often leads to nausea or other gastrointestinal side effects, and cannabinoids cannot penetrate the skin without the added help of permeation technology. Because Zygel can be absorbed through the skin, the product overcomes these challenges. If Zygel is found to be effective in treating FXS, Zynerba may be able to pioneer similar transdermal therapies for multiple conditions.