NextUp: The Malvern Medtech Company Working to Improve Surgical Mesh Options
TELA Bio is striving to reduce the adverse effects of hernia repair with OviTex, their biological material-based surgical mesh product.
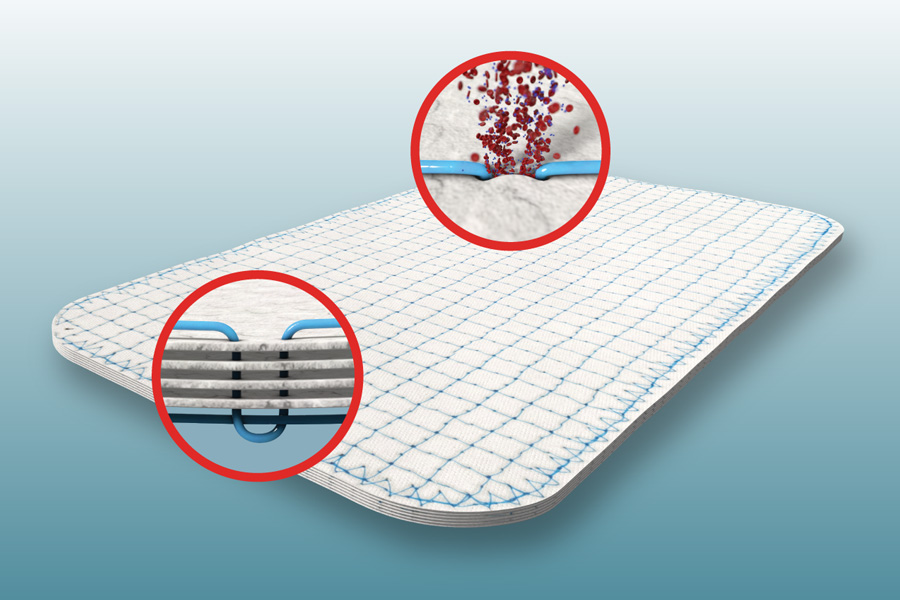
TELA Bio’s OviTex uses a lockstitched polymer through the layers of biologic material to allow for a product that’s both permeable and strong. Image courtesy Tela Bio
“NextUp” is a weekly NextHealth PHL feature that highlights the local leaders, organizations and research shaping the Greater Philadelphia region’s life sciences ecosystem. Email ccunningham@phillymag.com with pitches for NextUp.
Who: Co-founders, president and CEO Antony Koblish and CMO Maarten Persenaire met around the turn of the millennium, when they were both working for Orthovita, a Malvern-based manufacturer of orthopedic biologics. When Orthovita was sold to Stryker Corporation in 2011, Koblish and Persenaire — who had developed a strong portfolio and professional relationship by that point — decided to start their own medtech business one year later.
What: Based in Malvern, TELA Bio is a commercial stage medical technology company focused on surgical reconstruction solutions. More specifically, they design and market soft-tissue reinforcement materials in order to improve existing biologics and reduce the negative physiological impact permanent synthetic material can have on patients.
Their recent focus has been developing and refining their surgical hernia mesh option, OviTex. OviTex is composed of layers of biologic material made of ovine rumen (a.k.a. part of the digestive tract of sheep and cows) that’s lockstitched together with polymer fiber that can either be resorbable or permanent, depending on patients’ needs. The permeable design of OviTex allows for blood and wound fluids to move through it, while the lockstitching helps optimize stretching and prevent ripping. And because it’s made primarily of a biologic material — the ovine rumen — rather than fully synthetic materials, it can reduce foreign body response when applied. Moreover, OviTex is fashioned as a textile using 3D printing, so TELA’s engineers can tailor the thickness and stretchability across patients. Currently, TELA Bio offers OviTex in its original design, plus four other configurations suited for a variety of hernia repairs, as well as plastic and reconstructive surgery.

TELA Bio co-founders Antony Koblish (left) and Martin Persenaire. Photographs courtesy Tela Bio
When: Koblish and Persenaire co-founded TELA Bio in 2012, then spent the next several years developing and testing prototypes of OviTex. According to Persenaire, these stages were done with over 100 surgeons to make sure the product was made and performed exactly how it should — with optimal patient safety in mind. “Whenever you’re designing and developing a product to be inserted into a human body, there are no shortcuts,” he says. In April of 2017, TELA Bio began clinical testing on OviTex’s effectiveness of mitigating postoperative complications and re-herniations. Thirty days following operations, none of the 85 evaluated subjects required surgical intervention or implant removal, and only 20 percent required minimal procedural intervention. Two years later, 20 patients opted into a volunteer follow up. Four had developed a minor infection within that time frame, but none had experienced a hernia recurrence.
At the end of 2019, TELA Bio went public, and in January of this year, they brought on Bruce Freedman, a private practice general surgeon, as vice president of clinical development. Freedman, who has over 32 years of experience in hernia repair and abdominal wall reconstruction, will work to connect with local surgeons and hospital administration in order to improve patient access to OviTex.
What it means: According to the FDA, more than one million hernia repairs are performed each year in the United States; however, an abundance of adverse effects — including hernia recurrence, pain, scar tissue adhesion, and infection — have been reported with now-recalled mesh products since the early 2000s. In other words, the area of soft tissue mesh products was ripe for new options.
OviTex is working to bring together the benefits and minimize the shortcomings of both biologic materials and synthetic materials, all while lowering costs associated with hernia repair. Overall, OviTex aims to reduce the amount of unintended consequences of surgical mesh, while still fulfilling its basic function: supporting and strengthening the area weakened by hernia.
Why it matters now: Though TELA Bio is not focused on creating products related to the novel coronavirus, they believe OviTex has emerged as a viable surgical device at an appropriate time. “Hospital systems are under stress and strain from the pandemic, and we think they need more cost-effective solutions for treating all patients, not just those coming in for COVID-19,” says Koblish. Plus, according to a report by Fior Markets, the global hernia repair market is expected to grow to $6.37 billion by 2025 due to increasing hernia incidences and obesity rates, plus a rising geriatric population and a demand for more cost-effective non-synthetic meshes. TELA Bio believes OviTex can help drive that growing market.